So I decided to conduct a research study to figure out which methoxy groups in different position isomers of NBOMes produce stronger results. In other words, I wanted to measure the potency and efficacy of different variations of the NBOMes. I thought this research would be fun to do due to the reason that this structure-activity relationship illustration has not been published yet.
For the sake of convenience and practicality the NBOMe derivative used for this experiment is going to be 25i-NBOMe due to its popularity and predominance in the drug market.
As a brief explanation to all the n00bs out there who don’t know the group positions of an NBOME I have made the following illustration:
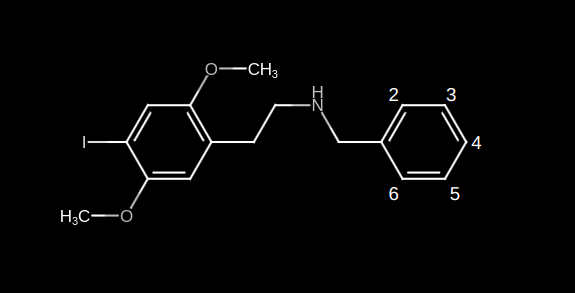
In the N-Benzyl group of the NBOMe there are 5 different group positions: 2, 3, 4, 5 and 6.
Usually all NBOMes have the methoxy group (hence the ‘OMe’) are saturated in position 6, like so:
I had never found a batch with any of the other group positions saturated so I wanted to see what happened if you saturated any of those other groups and which ones produce stronger effects.
In a quest to determine this question, the following 5 isomers of 25i-NBOMe were mysteriously synthesized and tested by me and a group of friends in a non-controlled environment:
The dosage for each test was of 300 μg. The results of the tests were the following:
Stronger potency means that the substance yields more visuals and more changes in the perception of reality.
It seams that for overall drug production, position 6 is preferred since position 3 results too much and a little unbearable for beginners, but as the user get used to the results position 3 becomes preferred due to its stronger efficacy.
Saturating more than 1 group at once results in very strong and intense effects that only highly advanced users can manage and bear. It does yield a whole other level of unfathomable unearthly experience.
