The intention of this post is to illustrate how strong are the effects of different variations of the very powerful tryptamine DMT. I also designed a new tryptamine called 4-MeO-BRMT and I published its molecular structure at the end of this post.
DMT is a tryptamine which means that it has the following molecular structure:
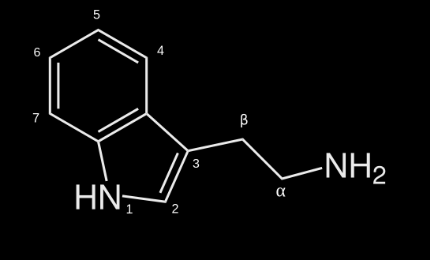
The different positions (1, 2, 3, 4, 5, 6, 7, a, b, N) can be saturated with different elements (functional groups) to form structural analogues (variations of DMT).
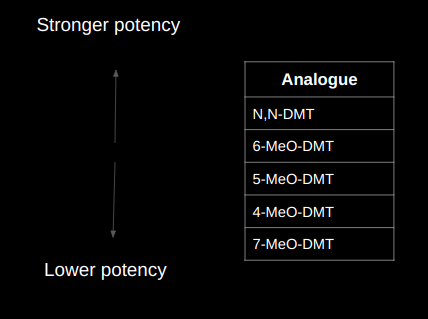
The following 5 structural analogues of DMT were synthesized and given to test-subjects in order to be compared:
- N,N-DMT
- 4-MeO-DMT
- 5-MeO-DMT
- 6-MeO-DMT
- 7-MeO-DMT
N,N-DMT
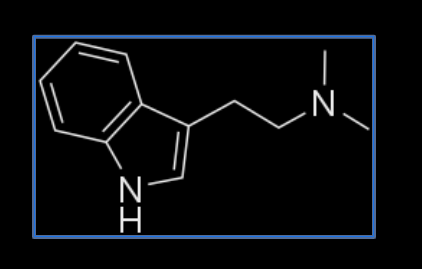
This form of DMT yields by far the strongest and most intense experience compared to the other DMT analogues. But really, the effects yielded by this molecule are not even comparable to the others. Users experience a very bold time-dilation: They claim they tripped for several hours, days, or even for an eternity, but when they come back only 5 minutes have passed in ordinary reality.
Users also claim that most of the experience offered by DMT is impossible to explain with words or to be understood without the altered state of consciousness produced by the drug.
This is clearly not the type of DMT that most people get. Although this is not publicly known, I think the substance available for the people to buy is 5-MeO-DMT which is extremely weaker. Although, scientifically speaking, the term DMT strictly refers to N,N-DMT, and not 5-MeO-DMT.
All DMT analogues are selective neurotransmitters, which means they don’t activate all 15 serotonin receptors (5-HT receptors) but only some of them. N,N-DMT is the least selective molecule so it binds to more receptors than any of the other DMT analogues.
4-MeO-DMT
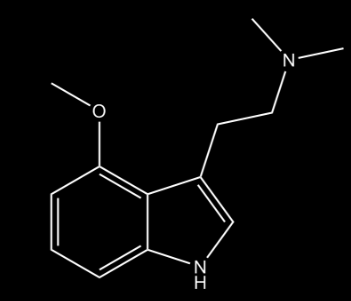
This DMT analogue creates a far less intense experience compared to N,N-DMT due to the fact that the MeO saturation in position 4 makes the molecule more selective.
The user describes the sensation of as being immersed in a dream. This dream-like experience is what distinguishes this variation of DMT. The structure and effect of this drug is very similar to psilocin (4-HO-DMT), which is the psychoactive substance present in psychedelic mushrooms, but the MeO substitute makes it a little bit stronger than the HO substitute..
The potency and intensity of this drug turned out to yield the less potent experience among all of the other structural analogues in this case-study.
5-MeO-DMT
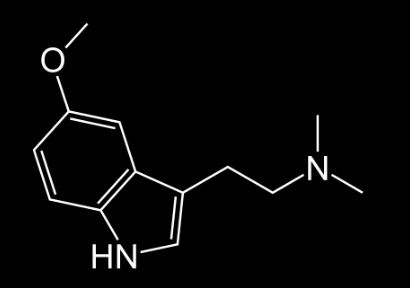
Regular DMT is not being sold to the people anymore because it is extremely powerful and the experience can be dangerous and damaging if the user does not have the awareness of what he is doing in the altered state of consciousness. This is why 5-MeO-DMT is being sold in the market presented as DMT. I’m sure that users that have been using DMT for a considerable long time noticed that they are not getting the same type of DMT than they used to.
Users report a stronger experience compared to the previous cases-study (4-MeO-DMT). They also claim that their vision is enhanced with everything being more colorful and considerably more detailed.
The experience is described as being way more lucid in contrast to the dream-like sensation of 4-MeO-DMT, and that they can understand things at a more profound level.
It might be worth mentioning that although serotonin is a non-psychedelic tryptamine, it is saturated with a HO group in position 5.
6-MeO-DMT
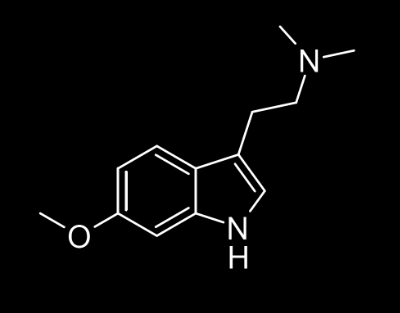
This turned out to be even more powerful than the two previous case-studies.
As the psychedelic effects of the experience are hard to describe in words, the only thing that can be said is that the experience is more significant that the other selective analogues.
7-MeO-DMT
This resulted in the weakest and mildest experience between all the structural analogues.
Saturation with different functional groups
Instead of using the MeO (methoxy) functional group, there are not too many different groups of elements that can be used instead to saturate the positions 4, 5, 6 and 7.
An HO (hydroxy) functional group can also be used and it results in a milder experience compared to MeO. In fact, 4-HO-DMT is the molecular structure of the very popular psilocin that can be extracted from psychedelic mushrooms but it can also be synthesized in a lab.
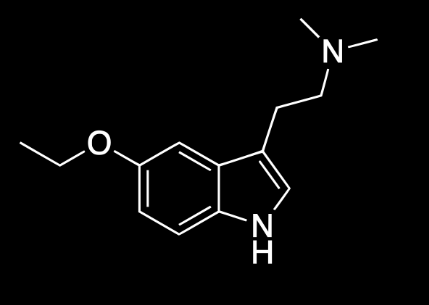
Besides HO, longer amino acid chains can be used, such as EtO (ethoxy). 5-EtO-DMT
Saturating positions 4,5,6,7 with stronger functional groups such as Br or even Cl is not a good idea because users claim they feel drowsy, disturbed and difficulty to think.
On the other hand, saturating position N with strong elements results in an immensely stronger psychedelic experience. Inexperienced users claim that it is too much. I haven’t seen such type of molecule documented anywhere, but I would call it 4-MeO-BRMT and it would look like this:
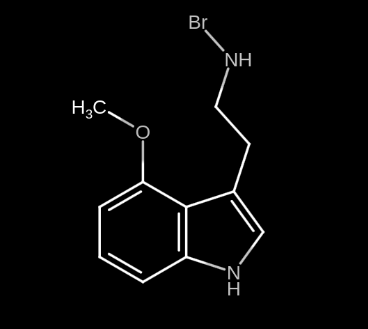
Notice how position 4 was saturated with an MeO group to make it more selective, this is to make the potency of the molecule not as intense. Also, notice how position N was saturated only with one Br group, instead of 2 Methyl groups as in DMT (dimethyltryptamine). I would consider this to be enough. Honestly I don’t think I could ever bear a tryptamine with 2 Br groups at position N.
However, I made a better version of BRMT and it looks like this:
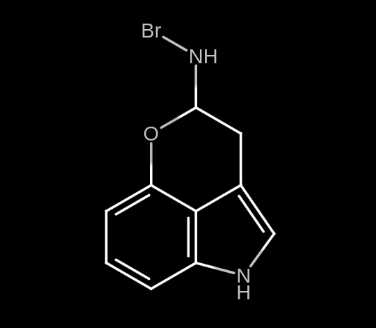
The suggested dose would be between 0.5 and 2 mg. I wouldn’t say this is a recreational experience, it rather is a tool for exploration of consciousness.
Saturating more than one numeric position.
If more than one numeric position (4, 5, 6, 7) is saturated, users get nauseated and dizzy because the guts have many serotonin receptors that are selective for tryptamines saturated at more than one of the mentioned numeric positions.
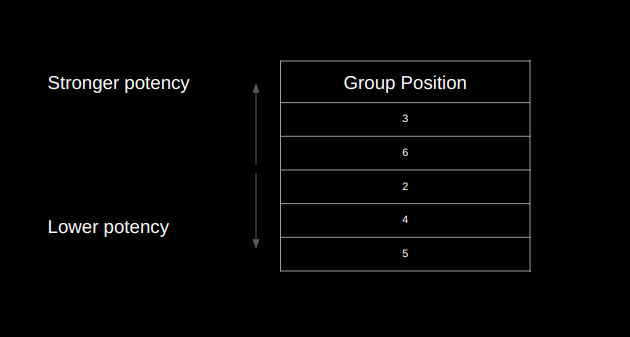
Results